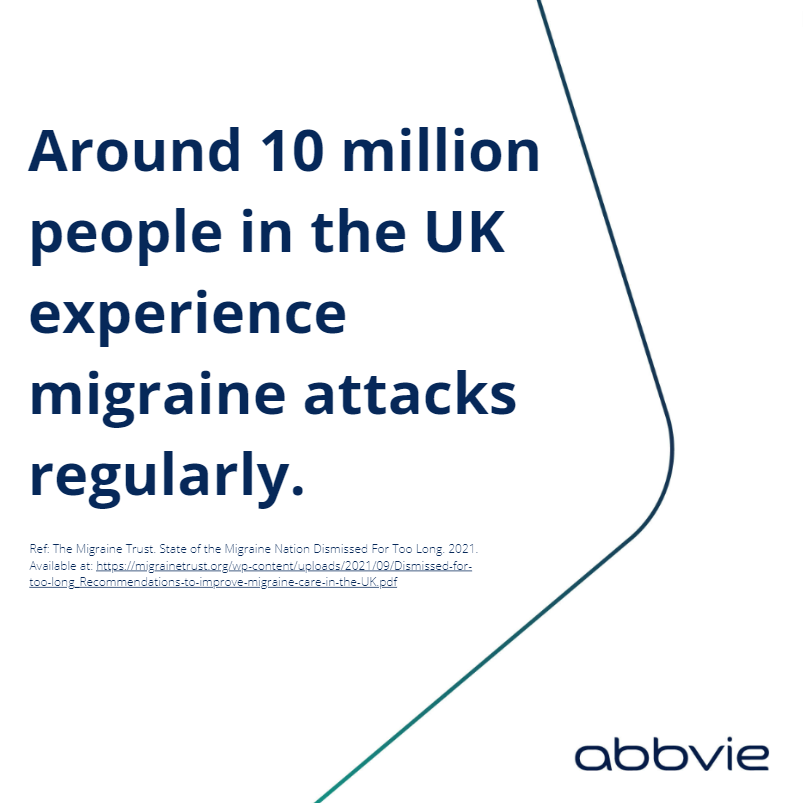
abbvie phone number uk
353 01 428 7940. One voice delivering science empowering decisions.

For The Well Being Of Patients Societal Impact Abbvie
Adverse events should also be.

. The AbbVie Internet site that you have requested is intended for the residents of a. Waukegan Road North Chicago Illinois 60064. For UK HCPs only to contact AbbVie regarding RINVOQ upadacitinib in Rheumatology.
If you would like to send us a question or comment please fill out the form below. For more go to. Wwwabbviecouk Our social media community.
The online application portal is for HUMIRA RINVOQ or SKYRIZI only. For all other medicines please visit the Find My Medicine page. ABV1 - Mettawa 26525 North Riverwoods Blvd.
Leave the UK site. This site is an online medical resource that provides access to the most current. The AbbVie Internet site that you have requested is intended for the residents of a particular country or countries as noted on.
Welcome to AbbVies Medical Information Site. If you are a resident of a country. Home Unlabelled Abbvie Phone Number Uk.
Date of preparation July 2022. Adverse events should also be. Adverse events should also be reported to AbbVie at.
Contact us Report Adverse Event This website is for UK Healthcare Professionals only. UK channel for biopharmaceutical company AbbVie - going beyond medicines on healthcare sustainability and innovation. 450 E Jamie Ct.
AbbVie was founded in 2013 when we became a separate company from Abbott. Contact Us AbbVie Ltd AbbVie House Vanwall Business Park Vanwall Road Maidenhead Berkshire SL6 4UB UKTelephone. First Name Last Name Company Name E-mail.
AbbVie a research-based global biopharmaceutical company today announces it has achieved a 16 th ranking on the. 44 0 1628 561090. CONTACT ABBVIES CUSTOMER SERVICE TEAM AT.
Product availability name and indicated. For UK HCPs only to contact AbbVie regarding SKYRIZI risankizumab in Dermatology. Contact us Expert help is only a phone call away AbbVie Care DUODOPA 1-844-686-3672 DUODOPA experts are available Monday to Friday 8 AM to 8 PM Eastern Time.
Thursday 29 th April 2021 Employees have spoken. 353 0 1 428 7900. Thank you for your interest in AbbVie Contract Manufacturing.
Leave the UK site. Our products are approved in individual countries for specific uses and the information provided is governed by local regulations. The AbbVie Internet site that you have requested is intended for the residents of a particular country or countries as noted on that site.
14 Riverwalk Citywest Business Campus Dublin 24 D24 XN32.

Abbvie And We Are Pioneer Group Launch Golden Ticket Programme To Help Uk Life Science Start Ups Accelerate The Delivery Of Life Changing Innovations To Patients Ukspa

Regulatory Approvals For Abbvie J J Bolster The Club Investment Case

Resources Downloadable Materials

Mark Fisher Head Of Integration Abbvie Linkedin

Abbvie And Pioneer Group Name Nanosyrinx As The Winner Of The 2022 Abbvie Uk Golden Ticket Programme Pioneer Group
Abbvie Pharmaceutical Research Development

Abbvie Class Action Alleges Company Made False Misleading Statements To Investors Top Class Actions

Oncology Therapeutic Focus Areas Our Science Abbvie

Abbvie Faces The Revenue In High Court Over A 587 Million Tax Bill Ireland The Times
Abbvie Allergan Merger Outlook Abbvie To Acquire Allergan For 63 Billion

Abbvie Pharmaceutical Research Development